Research
Aims
The primary objectives include:
- Replication of the structural characteristics of normal and malignant tissue using 3D bioprinters to reproduce the three-dimensional tissue architecture in the human body as closely as possible. In contrast, current standard methods are only insufficiently capable of reproducing the physiological situation in the body, especially the two-dimensional cultivation of cells on plastic.
- Development of test models for patient-specific drug testing to support physicians in treatment decisions. Ultimately, we aim to generate tissue surrogates to establish tissue replacement and regeneration strategies.
- To reduce animal testing or – in the long run – replace it wherever possible by providing suitable alternatives. Thus, the research facility is a proud partner of the MUI animalFree research cluster and a member of the RepRefRed Society. Further information can be found: Further information can be found
Research Projects
Antonia Degen: 3D-bioprinted tissue to study β-oxidation defects
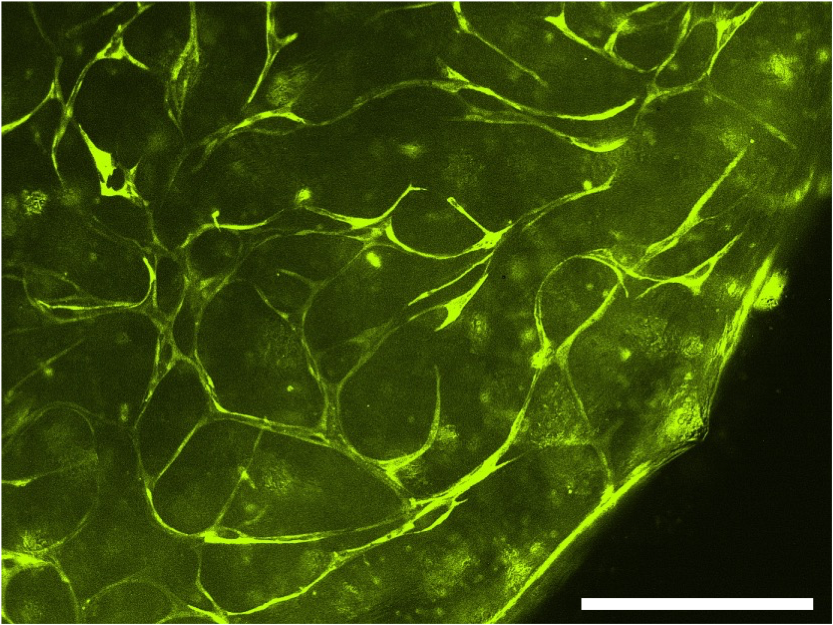
In this project, we are working with patient-derived cells from patients with Long-chain-3-hydroxy-acyl-CoA-dehydrogenase-deficiency (LCHADD) and Very-long-chain-acyl-CoA-dehydrogenase-deficiency (VLCADD). LCHADD and VLCADD are rare disorders of the oxidation of long-chain fatty acids. We are interested in the mitochondrial dysfunction of these β-oxidation-defective fibroblasts and study mitochondrial rearrangement by exposing them to various rescuers in 2D cell culture. To better mimic the tissue physiology we are establishing a fully 3D-bioprinted vascularized tissue model with healthy and patient-derived fibroblasts. These patient tissue models will be used for testing novel treatment modalities and tissue response during physiologic stress. Since cardiac complications are the leading cause of early and sudden death in LCHADD/VLCADD-patients, this study additionally aims to model these complications using human induced pluripotent stem cell (iPSC) technology. Therefore, patient-derived defective fibroblasts will be reprogrammed into iPSCs and differentiated into cardiomyocytes to study patient-related differences and the effect of different treatment modalities.
Alexeja Kleiter: 3D-bioprinted melanoma on-chip
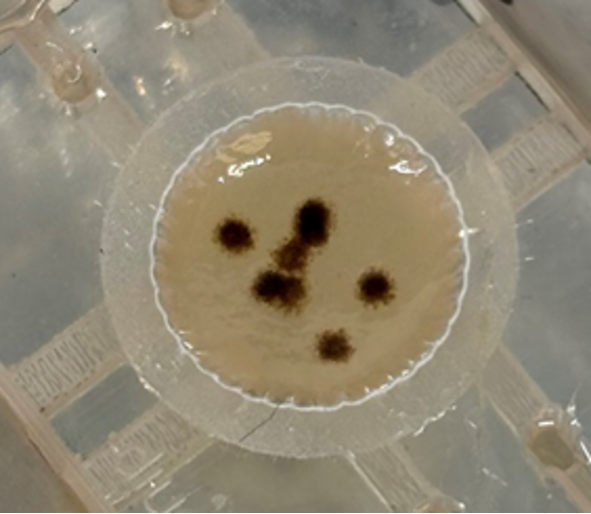
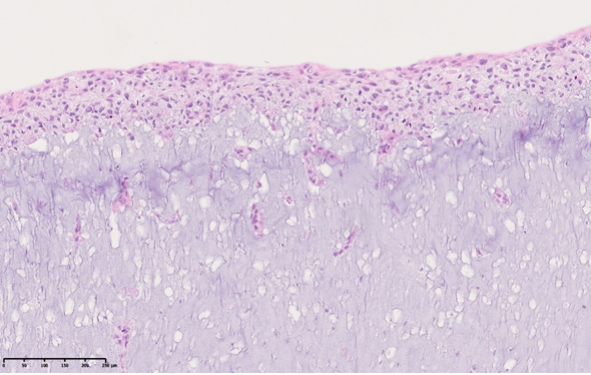
Melanoma remains one of the most aggressive skin cancers, and understanding tumor cell interactions with the immune system is crucial for developing effective treatments. However, tumor immunology research still heavily relies on mouse models, but translating these findings to patients is challenging. To address this, we focus on developing a human 3D-bioprinted melanoma-on-chip model including dendritic cells (DC) and Langerhans cells which play a central role in immune defense and anti-tumor immunity in the skin. As part of this effort, we successfully developed a microfluidic chip that provides stable and controlled environments for the cultivation of skin tissue and allows the incorporation of human melanoma spheroids. To investigate the interactions between melanoma cells and immune cells, CD34+ hematopoietic stem cells are differentiated into different DC subtypes and Langerhans cells. For the differentiation process, we could successfully replace fetal bovine serum with human AB-serum, which enhances the human relevance of the model while reducing reliance on animal-derived components. This platform represents a significant step forward in studying skin immunology and melanoma, offering new opportunities for investigating tumor-immune dynamics and testing therapeutic approaches.
Developing 3D-bioprinted vascularized neuroblastoma-on-chip model to study metastasis
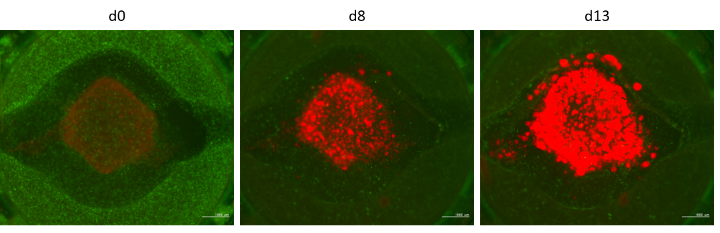
The progression of neuroblastoma, the most common extra-cranial pediatric malignancy, relies on shaping the tumor microenvironment (TME) and stimulating vascularization. We are developing a 3D-bioprinted model to recapitulate neuroblastoma TME on a fluidic chip by combining cancer cells with fibroblasts, immune cells, and blood vessels. This ‘tumor-on-chip’ will be connected to other tissue surrogates, such as the liver and kidney, in a custom-developed perfusion system. This screening platform can thus be used to simultaneously investigate the activation, metabolization and elimination of drugs via liver and kidney cells, as well as their influence on tumor growth and metastasis. This project primarily focuses on modulators of the transcription factor FOXO3 that plays a pivotal role in the oncogenesis and chemoresistance of neuroblastoma. Ultimately, this multi-organ tumor-on-chip model should serve as a platform to enable personalized therapeutic testing.
Verena Sturmlehner: Mesothelium-on-Chip system to study Ovarian Cancer
Ovarian cancer is the most lethal gynecological malignancy and the fourth-common cause of cancer-related death in the western world. One reason for its high mortality is that in more than 50% of ovarian cancer patients the ascites contains multicellular aggregates, which are likely to cause metastasis and relapse of the cancer. Therefore, this project focuses on the development of an advanced Mesothelium-on-Chip model to mimic angiogenesis, lymph vessel formation and mechanical forces to efficiently simulate the invasion of ovarian cancer spheroids into the mesothelium. This system will support the prediction of individual patients’ therapy response and has unmet potential for testing novel therapeutics.
Replacement-Reduction-Refinement of Animal Experiments [3R principle]
It is one of our main interests to reduce or replace animal experiments in biomedical research. We believe that with our innovative bioprinting-based tissue engineering and human Tissue-on-Chip models we will significantly contribute to the development of techniques to reduce animal experiments in biomedical research and drug testing. Therefore we are partners of the “MUI animalFree research cluster” at Medical University Innsbruck and member of RepRefRed Society.
Most biomedical applications and therapies have to be tested in animal experiments before they may be used in patients. However, animal testing must always be preceded by a comprehensive analysis of cell biological processes and drug effects in cell cultures. The better these models reproduce the natural tissue or tumor tissue, the better the experiments can be transferred to the test animal (or patient). Conventional 2D cell culture on plastic insufficiently reflects the physiological situation in the body. In contrast, 3D bioprinted tissue surrogates mimic the function or therapy-response of tissues more accurately. By using such advanced bioprinted models, animal experiments can be significantly reduced (reduction), drug concentrations can be estimated more precisely in advance (refinement) and, in the best case, animal experiments can be completely replaced (replacement).
Highlights
FFG/EFRE Infrastructure Grant ‘2Photon Polymerisation 3D Printing Facility at the Medical University of Innsbruck’ (project management Judith Hagenbuchner, Michael Ausserlechner)
MyPoint MUI, Projekte FFG
Start of the MUI-funded PhD programme CONNECT (Judith Hagenbuchner, Michael Ausserlechner)
MyPoint MUI
Daniel Nothdurfter receives ‘Otto Seibert Prize’
MyPoint MUI
Michael Ausserlechner is appointed ‘Univ. Professor for Tissue Engineering’ at the Medical University of Innsbruck
MyPoint MUI
Federal Minister Martin Polaschek awards the ‘State Prize for Alternatives to Animal Experiments 2022’ to Michael Ausserlechner.
MyPoint MUI
Bioprinting Team at MUI develops unique “tumor-on-chip” model for drug testing and precision medicine applications
Biofabrication, MyPoint MUI, Tiroler Tageszeitung, Salzburger Nachrichten, STOL.IT, zm online, europe-cities.com, studium.at, ORF.at, openscience.or.at, Pflege-Professionell.at, medinlive.at, APA Science, Innovationorigins, Kleine Zeitung
Kiwanis Prize awarded to Michael Ausserlechner October 2022
Lienz: Tiroler Kiwanis Preis ging an Forscher aus Osttirol
Michael Ausserlechner receives Research Grant from Dr. Johannes und Herta Tuba Fundation
MyPoint MUI
Judith Hagenbuchner develops novel 3D bioprinted tissue model for research on genetic fatty acid oxidation disorders as member of the Austrian Science Fund Research Group FG15
MyPoint MUI
Blog-Interview mit Michael Ausserlechner “Der Mensch aus dem 3D Drucker?”
medsolut.com
Interview of Judith Hagenbuchner with eco.nova
eco.nova (Seite 74ff)
Judith Hagenbuchner receives the Otto Seibert Science Award 2020
MyPoint MUI
The Federal Ministry Republic of Austria Education, Science and Research funds innovative 3D bioprinted Tumor-on-chip model by Judith Hagenbuchner
MyPoint MUI